Introduction:Elevated levels of the iron regulator hepcidin can cause functional iron deficiency anemia. Hepcidin dysregulation is central to anemia of chronic inflammation observed in several malignancies including myelofibrosis (MF). Activin receptor-like kinase-2 (ALK2 [ie, ACVR1]) contributes to MF-associated anemia via hepcidin upregulation. We evaluated the safety, efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of zilurgisertib-a potent and selective oral ALK2 inhibitor-in patients (pts) with anemia due to MF.
Methods:This ongoing open-label, multicenter, phase 1/2 dose-escalation/expansion INCB 00928-104 study (NCT04455841) is evaluating zilurgisertib alone (treatment group A [TGA]) or with ruxolitinib (RUX; treatment group B [TGB]). Eligible pts are ≥18 years old with primary/secondary MF of intermediate (Int)-1 (TGB only) or Int-2/high-risk (TGA and TGB) per the Dynamic International Prognostic Scoring System (DIPSS) and with anemia, including pts who are transfusion dependent (≥4 units of red blood cell transfusions during the 28 days or 8 weeks before Cycle [C] 1 Day [D] 1 for hemoglobin [Hb] <8.5 g/dL in the absence of bleeding or treatment-induced anemia) or are nontransfusion dependent with symptomatic anemia (Hb <10 g/dL during screening on 3 separate occasions ≥7 days apart and not meeting criteria for transfusion dependence). The zilurgisertib starting dose in TGA was 50 mg once daily (qd), with dose increases of ≤2-fold performed until a grade ≥2 toxicity with reasonable probability of being related to the treatment group was observed; subsequent dose increases were limited to ≤50% until the maximum tolerated dose (MTD) was reached or the recommended doses(s) for expansion were identified. In TGB, pts were on a stable regimen of RUX for ≥12 weeks before initiating zilurgisertib at 100 mg qd. The primary endpoints are safety and tolerability, including determination of dose-limiting toxicities (DLTs) and MTD. Secondary endpoints include efficacy (per anemia response parameters), PK, and PD (hepcidin measurement).
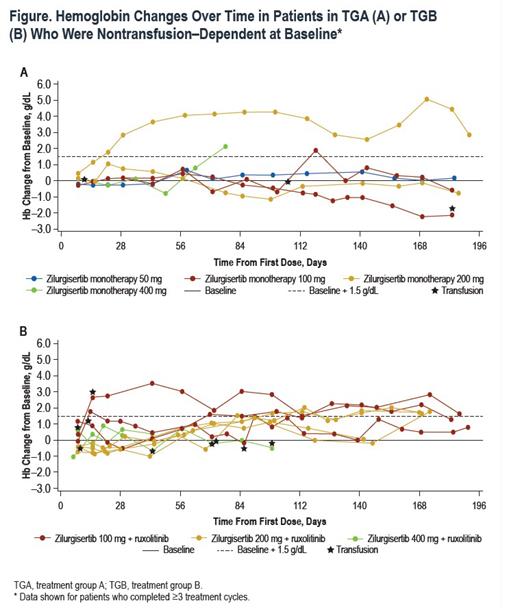
Results:A total of36 pts were enrolled at the time of analysis (data cutoff, February 15, 2023), with 20 in TGA and 16 in TGB. Median (range) age was 73 (53-84) years for TGA and 75.5 (54-85) years for TGB; 65% of TGA pts had received prior RUX therapy. Overall, 89% had Int-2 risk DIPSS, and the remainder had high-risk DIPSS (1 in TGA, 3 in TGB). In total, 55% of pts in TGA and 25% in TGB were transfusion dependent at time of enrollment. Median (range) baseline Hb was 7.7 (7-10) g/dL in TGA and 8.0 (5-9) g/dL in TGB. Baseline median (range) hepcidin was high in both cohorts (TGA, 171 [18-535] ng/mL; TGB, 126 [7-421] ng/mL; normal range, 0-50 ng/mL). At the time of analysis, dose escalation was ongoing in both treatment groups. No DLTs occurred in either treatment group, and the MTD had not been reached. Treatment-emergent adverse events (TEAEs) were mainly low grade without apparent dose dependence. Grade ≥3 TEAEs occurred in 11 pts, with thrombocytopenia the most common (n=4). The PK profile for zilurgisertib at steady state was consistent with that observed with previous healthy volunteer studies, with observed time of maximum concentration at 2-4 hours after administration and predicted half-life of approximately 24 hours. Maximum reduction in hepcidin following zilurgisertib dosing in C1D1 was observed at 6-8 hours postdose; the highest doses tested in both TGA and TGB demonstrated more effective maintenance of hepcidin suppression over time compared with lower doses. Among non-transfusion-dependent pts, anemia improvement (Hb increase of ≥1.5 g/dL vs baseline) was observed in both treatment groups (Figure). Among transfusion-dependent pts, anemia response of transfusion independence had not been observed at data cutoff. All pts with anemia improvement have maintained Hb increase in the absence of transfusion and remain on study.
Conclusions:Treatment with zilurgisertib monotherapy or in combination with RUX in this pt population was generally well tolerated, with predominantly grade 1/2 TEAEs. Reduced hepcidin levels were observed at all dose levels with both monotherapy and in combination with RUX, with greater control of hepcidin over time observed at higher zilurgisertib doses. Preliminary improvements in anemia were observed in non-transfusion-dependent pts during dose escalation, suggesting potential for therapeutic activity.
OffLabel Disclosure:
Mohan:Karyopharm, Astex, Incyte, Kartos, Ichnos, NCCN: Research Funding. Oh:Protagonist: Membership on an entity's Board of Directors or advisory committees; Morphic: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Constellation/Morphosys: Membership on an entity's Board of Directors or advisory committees; Cogent: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology/GSK: Membership on an entity's Board of Directors or advisory committees. Kiladjian:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie, AOP Health, Bristol-Myers Squibb, GlaxoSmithKline, Incyte, Novartis, Pharmaessentia.: Consultancy. Gotlib:Abbvie, Blueprint Medicines Corporation, BMS, Cogent Biosciences, Incyte Corporation, and Protagonist Therapeutics: Research Funding. Ritchie:Celgene: Other: Travel Support, Speakers Bureau; Astellas Pharma, Jazz Pharmaceuticals, NS Pharma: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy, Other: travel, Research Funding; Novartis: Consultancy, Other: Travel Support, Research Funding, Speakers Bureau; Ariad: Speakers Bureau; Celgene, Incyte Corporation, Novartis: Consultancy. Guglielmelli:Novartis: Other: Other member of advisory board, speaker at meeting, Speakers Bureau; GSK: Speakers Bureau; Abbvie: Other: Other member of advisory board, speaker at meeting, Speakers Bureau. Hunter:Sierra Oncology: Membership on an entity's Board of Directors or advisory committees. Palandri:Novartis, BMS, Celgene, GSK, Amgen, AbbVie, Karyopharm, AOP, Sierra Oncology, Janssen: Consultancy, Honoraria. Boyer:Novartis: Membership on an entity's Board of Directors or advisory committees. Rambaldi:Abbvie: Honoraria. Mori:Novartis: Honoraria. Ito:BMS, Chugai: Honoraria, Research Funding; Asahikasei, Kyowa Kirin,: Research Funding; Mundipharma, Novartis, Takeda, AbbVie, Nippon Shinyaku, Eisai, CSL Behring, Sanofi: Honoraria. Lamothe:Incyte: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Yang:Incyte: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Cui:Incyte: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Seguy:Incyte: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. McBride:Incyte: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Bose:Incyte, BMS, CTI, Morphosys, Blueprint, Cogent, Sumitomo: Honoraria, Research Funding; GSK, Novartis, Karyopharm, AbbVie, Pharma Essentia, Jubilant, Morphic: Honoraria; Kartos, Telios, Ionis, Disc, Janssen, Geron: Research Funding.
zilurgisertib is a potent and selective oral ALK2 inhibitor being evaluated in patients with myelofibrosis and anemia